FDA BRIEF: Week of June 6, 2016

VAXCHORA (Cholera Vaccine, Live, Oral) Suspension for Oral Administration
PaxVax Bermuda Ltd., Hamilton, BERMUDA.

INDICATION: Active immunization against disease caused by Vibrio cholerae serogroup O1 in adults 18 through 64 years of age traveling to cholera-affected areas
UNMET NEED:
- Cholera, caused by Vibrio cholerae bacteria, acquired by ingesting contaminated water or food; profuse diarrhea, vomiting, dehydration; potentially life threatening
- Serogroup O1 is the predominant cause of cholera globally
- Need for additional cholera-prevention measures beyond preventive strategies recommended by CDC
REG PATHWAY: Fast Track, Tropical Disease Priority Review
MECHANISM OF ACTION: Live attenuated cholera bacteria that replicate in gastrointestinal tract; rises in serum vibriocidal antibody 10 days after vaccination
EFFICACY:
- Randomized, double-blind, V. cholerae challenge study in US (N=197), no prior history of cholera infection, VAXCHORA vs. placebo
- Endpoint: Lack of occurrence of moderate to severe diarrhea
- With VAXCHORA, 90.3% , 10 days postvaccination, 79.5% three months postvaccination vs 39% with plabeo
- Immunogenicity (2 studies): Vibriocidal antibody assay to measure serum levels of neutralizing antibodies. >90% post vaccination
SAFETY:
- Most common adverse reaction: Tiredness, headache, abdominal pain, nausea/vomiting, lack of appetite and diarrhea
LIFESTENT VASCULAR SYSTEM
Bard Peripheral Vascular, Inc. Tempe, AZ, USA

INDICATION FOR USE: Improve luminal diameter in the treatment of symptomatic de novo or restenotic lesions up to 240 mm in length in the native superficial femoral artery (SFA) and popliteal artery with reference vessel diameters ranging from 4.0 – 6.5 mm
REG PATHWAY: PMA Supplement
- The original PMA approved in 2009 indicated to improve luminal diameter in the treatment of symptomatic de novo or restenotic lesions up to 160 mm in length in the native superficial femoral artery (SFA) and/or proximal popliteal artery with reference vessel diameters ranging from 4.0 – 6.5 mm
- Supplement to expand indication to include treatment of lesions in mid and distal popliteal artery
DEVICE DESCRIPTION:
- LifeStent® Vascular Stent (stents 20 – 80 mm)
- LifeStent® XL Vascular Stent (stents 100 – 170 mm)
- Bard® LifeStent® Solo™ Vascular Stent System
- All contain self-expanding, flexible, nitinol stents that expand to a preset diameter upon exposure to body temperature
- Sents equivalent to one another in design; in addition LifeStent® and LifeStent® Solo™ stents contain 6 tantalum radiopaque markers on both the distal and proximal ends
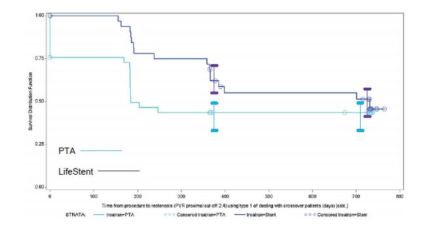
EFFECTIVENESS:
- ETAP trial, a prospective, randomized, multi-center study (n=246)
- LifeStent® vs. percutaneous transluminal angioplasty (PTA) in the treatment of patients with stenosis and occlusion of the popliteal artery .
- Primary endpoint: Primary patency, defined as freedom from target-lesion restenosis (luminal narrowing of ≥50%) as detected by duplex ultrasound. Lower restenosis rate at 12 months; followup for 24 month with Lifestent
- Secondary endpoint: Beneficial clinical trend in favor of stent placement with Lifestent
Freedom from Restenosis for Popliteal Segment 1
SAFETY:
- Major adverse events: Death, major amputation and minor amputation, TLR (including need for surgical revascularization), and myocardial infarction similar between two groups
- No major adverse events attributed to the device or procedure