FDA BRIEF: Week of October 31, 2016

By: Sheri Walker, Ph.D., FDA Senior Economist, and Clark Nardinelli FDA Chief Economist

FDA-regulated products account for about 20 cents of every dollar of annual spending by U.S. consumers.
- > $2.4 trillion in annual consumption (medical products, food, tobacco)
- Based on expenditure data collected by the Bureau of Economic Analysis (BEA)
How to Treat Impetigo and Control This Common Skin Infection
Causes:
- Staphylococcus aureus and Streptococcus pyogenes, bacteris found on skin
- > 3 million cases in US every year
- Prevalent in kids 2 – 6 years old: itchy red sores, itchy rash, fluid-filled blisters
Treatment:
- Presription topical or oral antibiotics
- NO over-the-counter (OTC) treatment
Performance of Drug and Biologics Firms in Conducting Postmarketing Requirements (PMRs) and Postmarketing Commitments (PMCs) (FY 2013 and FY 2014)
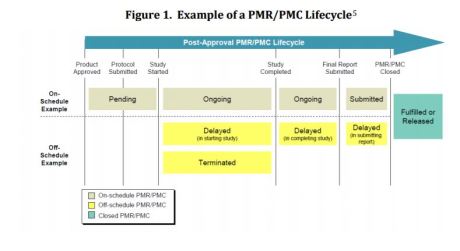
Postmarketing requirement (PMR): Drug study equired by statute or regulation to conduct after drug approval
Postmarketing commitment (PMC): Agreed post-approval study but not required by statute or regulation.
- To assess a known serious risk or identify an unexpected serious risk
- Study certain new drugs for pediatric populations
- Verify and describe the predicted effect for accelerated approval
- For approval on animal efficacy because human trials not ethical or feasible
CDER’s 2013-2014 Evaluation of PMRs & PMC Data
- Similar number of applicants and unique applications with PMRs/PMCs: more with drugs than biologics
- Most applicants meeting annual reporting obligation
- >50% PMRs for pediatric research
- Majority of PMRs/PMCs progressing on schedule
- Comparatively fewer PMRs/PMCs off schedule
National Cyber Security Awareness Month: Understanding the Interdependencies of Medical Devices and Cybersecurity

FDA guidances for monitoring, identifying, and addressing pre- and post-approval cybersecurity vulnerabilities in medical devices
- Life cycle approach to cybersecurity risk management – from early product development and extending throughout product’s lifespan
- Collaborating with entities that discover threats or vulnerabilities
- Developing appropriate solutions prior to vulnerabilities being publicly disclosed
FDA partnership with the National Health Information Sharing and Analysis Center (NH-ISAC), and the Medical Device Innovation, Safety, and Security Consortium (MDISS)
- Rapid sharing of medical device vulnerabilities, threats, and mitigations within the hospital and health care ecosystem
