FDA BRIEF: Week of December 26th, 2016 and January 2nd, 2017

ULTRAVISION™ Visual Field Clearing System
Alesi Surgical, Cardiff, UK
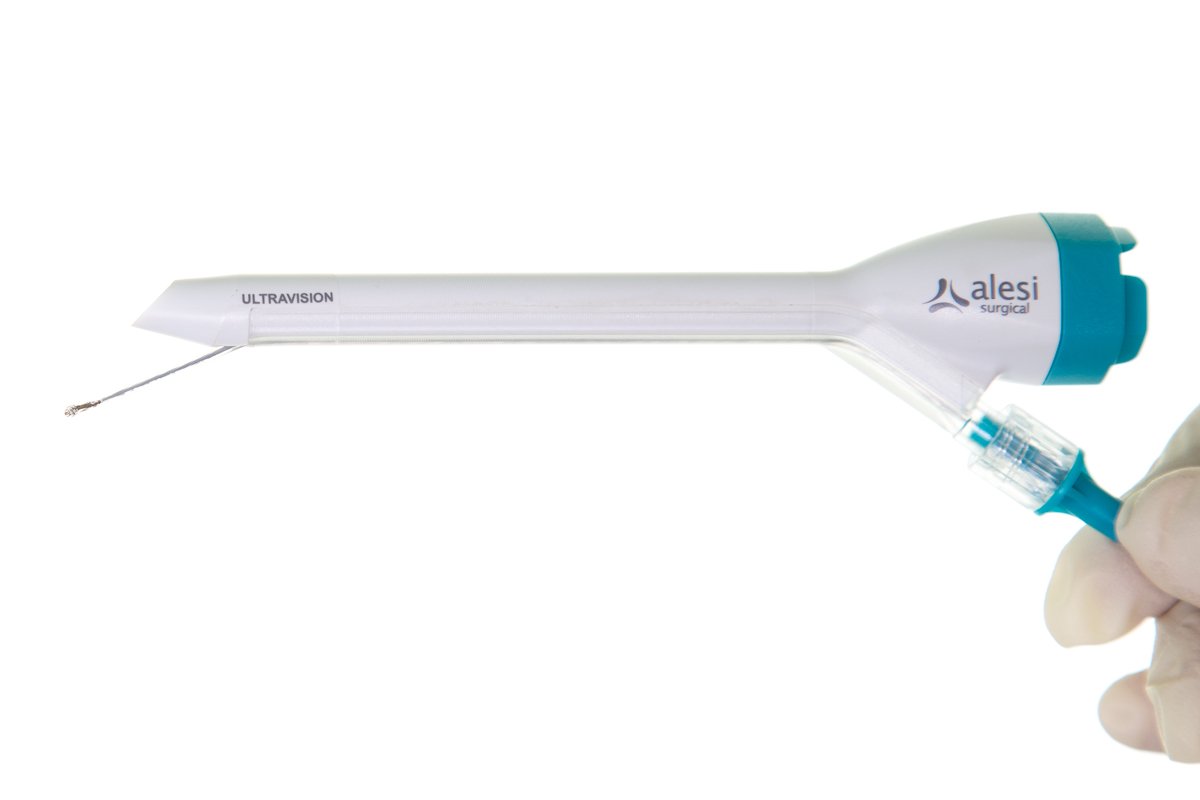
INDICATION FOR USE: Clearance of smoke and other particulate matter that is created during laparoscopic surgery.
REG PATHWAY: De Novo Request
- Regulation Number: 21 CFR 878.5050
- Regulation Name: Surgical smoke precipitator
- Regulatory Classification: Class II
- Product Code: PQM
GENERIC DEVICE TYPE: Surgical smoke precipitator
- Prescription device intended for clearance of the visual field by precipitation of surgical smoke and other aerosolized particulate matter created during laparoscopic surgery.
IDENTIFIED RISKS & MITIGATION:
- Electrical shock: Electrical safety testing. Labeling
- Electromagnetic interference: Electromagnetic compatibility testing, Labeling
- Infection Sterilization validation : Shelf-life validation, Labeling
- Adverse tissue reaction: Biocompatibility evaluation
- Tissue injury: Animal testing, Software verification, validation, and hazard analysis, Labeling
CLEANCISION Wound Retraction and Protection System
Prescient Surgical, San Carlos, CA, USA

INDICATION FOR USE: For use by a surgeon during abdominal surgery to retract the surgical incision, provide access to the abdominal cavity, and irrigate the surgical wound edge. The device may aid in the prevention of wound edge contamination. This device is intended to deliver a sterile irrigant solution and serve as a conduit for fluid removal from the surgical wound edge.
- Regulation Number: 21 CFR 878.4371
- Regulation Name: Irrigating Wound Retractor Device
- Regulatory Classification: Class II
- Product Code: PQI
GENERIC DEVICE TYPE: Irrigating Wound Retractor Device
- An irrigating wound retractor device is a prescription device intended to be used by a surgeon to retract the surgical incision, to provide access to the surgical wound, to protect and irrigate the surgical wound, and to serve as a conduit for removal of fluid from the surgical wound.
IDENTIFIED RISKS & MITIGATION:
- Adverse Tissue Reaction: Biocompatibility Evaluation
- Tissue or Wound Damage: Non-clinical Performance Testing, Shelf Life Testing, Labeling
- Infection: Sterilization Validation, Non-clinical Performance Testing, Shelf Life Testing, Labeling

SPINRAZA (nusinersen) injection, for intrathecal use
Biogen Inc. Cambridge, MA, USA

INDICATION: Treatment of spinal muscular atrophy (SMA) in pediatric and adult patients
UNMET NEED:
- SMA is hereditary, causes weakness and muscle wasting
- Wide variability in age of onset, symptoms and rate of progression
- Long-standing need for a treatment
REG PATHWAY: NDA. Fast track designation, Priority Review, Orphan Drug Designation, Rare Pediatric Disease priority review voucher
MECHANISM OF ACTION: Antisense oligonucleotide (ASO) designed to treat SMA caused by mutations in chromosome 5q that lead to SMN protein deficiency.
EFFICACY:
- Multicenter, randomized, double-blind, sham-procedure controlled, n =121, symptomatic infants ≤ 7 months, SPINRAZA vs. sham injection
- Primary endpoint (interim analysis): Responders with improvement in motor milestones according to Section 2 of the Hammersmith Infant Neurologic Exam (HINE)
- Interim Analysis: 21 (40%) vs. 0 (0%), p<0.0001
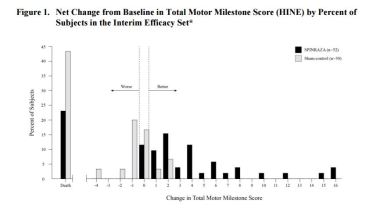
SAFETY:
- Most common side effects: Upper respiratory infection, lower respiratory infection and constipation
- Warnings and Precautions: Low blood platelet count, renal toxicity, neurotoxicity