FDARA & User Fees

FDARA and FDA User Fee Programs
President signed into law the Food and Drug Administration Reauthorization Act (FDARA)
- Revises and extends FDA’s user-fee programs for prescription drugs (PDUFA), medical devices (MDUFA), generic drugs (GDUFA) and biosimilar (BsUFA) products
- Provides FDA resources for increasing regulatory efficiency and expedite availability of innovative, safe and effective medical products
- Read how FDARA is making a difference
Drug User Fees
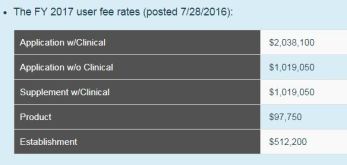
Medical Device User Fees
