

Procedures for Meetings of the Medical Devices Advisory Committee
Background and Scope:
- Provide information on processes for Medical Devices Advisory
Committee meetings - Excludes Medical Devices Dispute Resolution Panel (DRP)
- Does not apply to Device Good Manufacturing Practice Advisory Committee,
National Mammography Quality Assurance Advisory Committee, Technical
Electronic Product Radiation Safety Standards Committee
Overview:
- Panel Meeting Topics
- Advice on a Premarket Submission
- Regulatory Issues: Classification/Reclassification, General Issues
- Panel Expertise
- Two or more voting members with relevant clinical expertise
- One voting member knowledgeable about device technology
- Preparation for Panel Meetings:
- Premarket Submission Meetings, Briefing Material Contents, CDRH-Applicant Interactions, Regulatory Issues Meetings
- Conduct of Panel Meetings
- Medical Device Industry Presentations
- CDRH Presentation
- Open Public Hearing
- Panel Deliberations and CDRH Questions
- Panel Voting
- Post Meeting Activities
- Teleconference Panel Meetings

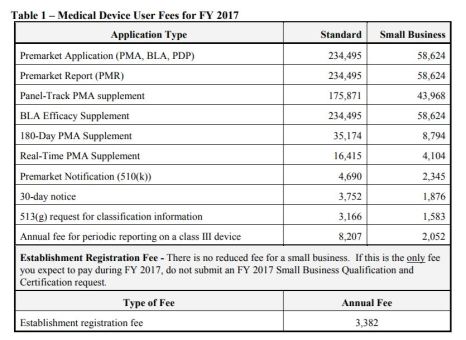
FY 2017 Medical Device User Fee Small Business Qualification and Certification
Background and Scope:
- Business qualified and certified as a “small business” is eligible for a substantial reduction in most of these user fees
- Process to request qualification and certification as a small business – US and Foreign
Overview:
- Eligibility
- U.S. Businesses
- Foreign Businesses
- National Taxing Authority
Small Business Fees:

Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices
Background and Scope:
- Real-World Data (RWD): Data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources
- Real-World Evidence (RWE): Clinical evidence regarding the usage, and potential
benefits or risks, of a medical product derived from analysis of RWD - Data derived from real world sources can be used to support regulatory decisions
Overview:
- Regulatory Context in Which RWE May be Used: General considerations, Application of IDE Requirements
- Characteristics of RWD: Relevance, Reliability (Data accrual, Data assurance)
- Examples Where RWE is Used
- Expanded Indications for Use
- Postmarket Surveillance Studies
- Post-Approval Device Surveillance as Condition of Approval
- Control Group
- Supplementary Data
- Objective Performance Criteria and Performance Goals


Design Considerations and Premarket Submission Recommendations for Interoperable Medical Devices
Background and Scope:
- Advancing the ability of medical devices to exchange and use information safely and effectively with other medical devices as well as other technology
- Potential to increase efficiency in patient care
- Promote development and availability of safe and effective interoperable
medical devices
Overview:
- Design Considerations for Interoperable Medical Devices
- Purpose of the Electronic Interface
- Anticipated Users.
- Risk Management Considerations
- Verification and Validation Considerations
- Labeling Considerations
- Use of Consensus Standards
- Recommendations for Contents of Pre-market Submissions
- Device Description
- Risk Analysis
- Verification and Validation
- Labeling

Evaluation and Reporting of Age, Race, and Ethnicity Data in Medical Device Clinical Studies
Background and Scope:
- Improve data quality, consistency, transparency regarding device performance within specific age, racial, and ethnic groups
- Can benefit patients, clinicians, researchers, regulators, and others
- Recommendations to overcome barriers to enrollment
Overview:
- FDASIA: Considerations for age-, race- and ethnicity-specific differences
- Participation of subgroups in clinical trials.
- Recommendations for Appropriate Enrollment
- Study Design, Early Enrollment Stage
- Premarket Submission Stage
- Postmarket Submission Stage
- Planning for Diverse Study Recruitment
- Study Design, Early Enrollment Stage
- Premarket Submission Stage
- Postmarket Submission Stage
- Considerations for Study Follow-Up Visits
- Interpretation of Study Results
- Assessing Heterogeneity Across Subgroups
- Recommendations for Subgroup Specific Statistical Elements
- Recommendations for Submissions to Agency