Week of October 16, 2017

YESCARTA™ (axicabtagene ciloleucel) suspension for intravenous infusion
Kite Pharma, Inc. (Gilead company)
INDICATION: Treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma.
Limitation of Use: Not indicated for the treatment of patients with primary central nervous system lymphoma
ADDRESSING UNMET NEED:
- DLBCL most common type of NHL in adults; 72,000 new cases of NHL diagnosed in the U.S. each year, and DLBCL represents approximately one in three cases
- Milestone in the development of a whole new scientific paradigm for the treatment of serious diseases
- Second gene therapy approved by the FDA; first for certain types of non-Hodgkin lymphoma (NHL)
REGULATORY PATHWAY: BLA
- Orphan Drug Designation; no pediatric requirements
- Postmarketing requirements: Assessment of long-term safety
- REMS requirements: Elements to assure safe use, implementation system
and timetable for submission of REMS assessments
MECHANISM OF ACTION: CD19-directed genetically modified autologous T cell immunotherapy, binds to CD19-expressing cancer cells and normal B cells. Studies demonstrated that following anti-CD19 CAR T cell engagement with CD19-expressing target cells leads to sequence of events killing of CD19-expressing cells
ADMINISTRATION: Each dose is a customized treatment created using a patient’s own immune system to help fight the lymphoma. The patient’s T-cells, a type of white blood cell, are collected and genetically modified to include a new gene that targets and kills the lymphoma cells. Once the cells are modified, they are infused back into the patient
EFFICACY:
- Single-arm, open-label, multicenter trial, n=100 patients with relapsed or refractory aggressive B-cell non-Hodgkin lymphoma, single infusion of YESCARTA
- Efficacy: Complete remission (CR) rate, duration of response (DOR)
- CR= 52 patients, median DOR= 9.2 months
SAFETY:
- Boxed Warning: Cytokine release syndrome (CRS) and neurologic toxicities that can be fatal or life-threatening
- Other side effects: Serious infections, low blood cell counts, weakened immune system. Side effects from treatment with Yescarta usually appear within the first one to two weeks
- REMS : Certification of hospitals and their associated clinics to recognize and manage CRS and nervous system toxicities
REIMBURSEMENT AND ACCESS:
- Available only in authorized treatment centers
- Ongoing and active discussions with payers
- Pricing based on discussions with government agencies that reimburse for drug costs and private insurers, and cancer centers.
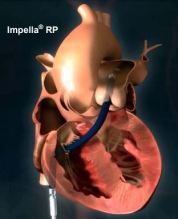
IMPELLA RP® System
ABIOMED, Inc.
INDICATION FOR USE: Providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥1.5 m2, who develop acute right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery
ADDRESSING UNMET NEED: Smallest heart pump for right heart failure
REGULATORY PATHWAY: PMA
- Regulation Number: 870.4360
- Product Code: PYX
- Postmarketing requirement: Real-world evidence on safety and effectiveness
DESCRIPTION: Comprised of three components
- Impella RP Catheter: a 22 Fr micro-axial flow pump catheter and its accessories
- Automatic Impella Controller (AIC): a reusable external drive console
- Impella Purge Cassette: an infusion pump used to flush the Impella RP Catheter
EFFECTIVENESS AND SAFETY:
- Pooled from the following three (3) datasets: Impella RP System pivotal study: 30 patients, Impella RP System continued access protocol (CAP) study: 4 patients, Impella RP System post-approval study (PAS): 26 patients
- Use of System to provide percutaneous hemodynamic support for right heart failure: Survival rate of 73.3% at 30 days
- Main adverse events: Major bleeding and hemolysis, no pulmonary embolism
REIMBURSEMENT FOR IMPELLA DEVICES:
- Hospital ICD-10 procedure code specific to percutaneous heart assist
- Separate physician payment for insertion, repositioning, and removal (CPT)
- Coverage by Medicare and major payers
Image Credits: Kite, Abiomed