FDA Brief: Week of Feb 15, 2016
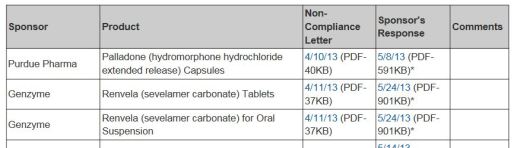
Non-Compliance Letters Listing
WHAT: Identifies drug and biologic products for which the sponsor has received a pediatric research Equity Act (PREA) Non-Compliance letter
WHY: Sponsors required to submit pediatric assessments required by PREA as part of drug approval
HOW: Provides links to the PREA Non-Compliance letter and sponsor response. When the sponsor fulfills, FDA will add the date to the last column of the table
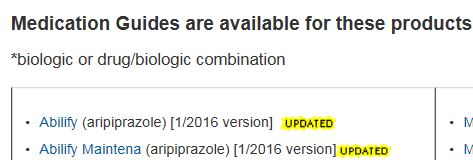
Medication Guide Database
WHAT: Paper handouts provided with certain prescription medicines. Contain information to avoid serious adverse events
WHY : FDA requires Medication Guides be issued when Agency determines that certain information is necessary to prevent serious adverse effects
HOW: Inform Patient decision-making by information about known serious side effect, or patient adherence to directions for the use