News & Views: 24th FDA Commissioner, Drug importation from Canada, Center of Excellence for drug compounding, Acute pain opioid prescribing, Generic drug pricing, Operation Vapor Lock, Naloxone and opioid overdose, 2019-2020 Flu season


Stephen Hahn, MD, confirmed as the 24th FDA Commissioner
- Radiation Oncologist with Residency at the National Cancer Institute
- Professor at University of Pennsylvania
- Chief Medical Executive at MD Anderson Cancer Center
Steps to lower U.S. prescription drug prices
Allow importation of certain prescription drugs shipped from Canada
- Purpose of proposed rule is to lower prices and reduce out of pocket costs
- Foreign seller, licensed by Health Canada and registered with FDA, to purchase directly from manufacturer
- US importer, subject to the supply chain security requirements, to buy directly from foreign seller
- Importer arranges for statutorily prescribed testing for authenticity, degradation, and other requirements by a qualifying US laboratory
- Post-importation requirements including adverse event, medication error, field alert to manufacturer and to FDA
 Improving quality of compounded drugs
Improving quality of compounded drugs
Novel approaches to reduce risks with production practices of outsourcing facilities
- Establishing Compounding Quality Center of Excellence to enhance collaboration among and provide educational programs for outsourcing facilities
- Three main areas of focus: in-person, online education and trainings, conference to exchange ideas and best practices, market research help inform FDA on key issues

 National Academies of Sciences, Engineering, and Medicine (NASEM) report on framing opioid prescribing guidelines for acute pain
National Academies of Sciences, Engineering, and Medicine (NASEM) report on framing opioid prescribing guidelines for acute pain
FDA contracted NASEM for evidence-based guidelines for opioid analgesic prescribing for acute pain. NASEM recommendations are:
- Develop an analytic framework (e.g., Figure above ) to develop and assess evidence base for clinical practice guidelines (CPG)
- Outpatient opioid prescribing CPGs should explicitly state patient populations (e.g., adults versus children) and contextual aspects (e.g. setting, prescriber type, prior treatments)
- To determine optimal opioid prescribing strategies, examine not only intermediate outcomes (e.g. pills prescribed, unused, long-term opioid use), but also the short- and long-term health outcomes (e.g. mortality, overdose, opioid use disorder, pain, and function)
- Well-designed observational and quality improvement initiatives helpful for evaluating the effects of opioid prescribing strategies on health outcomes
- CPGs should be implemented by governmental (federal, state, and local) and nongovernmental entities
- Prioritized surgical and medical indications listed for CPG development
- State the role of opioid alternatives as first-line therapies, specify any other interventions, including nonopioid interventions, used to relieve pain
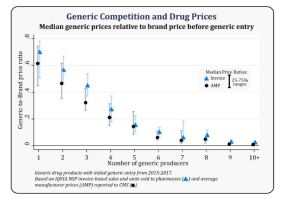 Greater Generic Competition and Lower Generic Drug Prices
Greater Generic Competition and Lower Generic Drug Prices
- New analysis showing greater competition among generic drug makers associated with lower generic drug prices
- With six or more competitors, price reductions of >95% compared to brand prices before generic entry
- FDA helping bring greater efficiency and transparency to generic drug review process to encourage competition
 Operation Vapor Lock seized sale of illicit THC vaping cartridges
Operation Vapor Lock seized sale of illicit THC vaping cartridges
FDA and DEA have seized 44 websites advertising the sale of illicit THC vaping cartridges
- Website advertising under various brand names with information indicating sale items would be considered a controlled substance under federal law
- Some websites solely to fraudulently obtain payments without intending to mail product
 Having Naloxone on Hand Can Save a Life During an Opioid Overdose
Having Naloxone on Hand Can Save a Life During an Opioid Overdose
Naloxone is a life-saving drug that, when sprayed into the nose or injected, quickly reverses the powerful effects of opioids during an overdose
- Expanded availability by allowing consumers to get directly from pharmacist, by putting a “standing order”
- Need to recognize opioid overdose and use Naloxone
- Will not harm if no opioids in system
- Discuss Naloxone when getting opioid prescription
 2019-2020 Influenza season
2019-2020 Influenza season
Flu vaccine lots that have been released by FDA and are available for distribution by the manufacturers
Image credit: FDA
