Device Authorizations: MYRIAD myChoice CDx diagnostic, PENTAX duodenoscope, MISIGHT contact lens, TULA system for otitis media

MYRIAD myChoice® CDx
Myriad Genetic Laboratories, Inc.
INDICATION FOR USE: Next generation sequencing-based in vitro diagnostic test that assesses the qualitative detection and classification of single nucleotide variants, insertions and deletions, and large rearrangement variants in protein coding regions and intron/exon boundaries of the BRCA1 and BRCA2 genes and the determination of genomic Instability Score (GIS) which is an algorithmic measurement of Loss of Heterozygosity (LOH), Telomeric Allelic Imbalance (TAI), and Large-scale State Transitions (LST) using DNA isolated from formalin-fixed p raffin embedded (FFPE) tumor tissue specimens.
The results of the test are used as an aid in identifying ovarian cancer patients with positive homologous recombination deficiency (HRD) status for treatment with the targeted therapy in accordance with the approved therapeutic product labeling.
ADDRESSING UNMET NEED: First diagnostic for testing of FFPE ovarian tumor tissue for the selection of patients who are eligible for treatment with niraparib (Zejula)
DEVICE DESCRIPTION:
- Single-site assay; includes reagents, software, instruments and procedures for testing DNA extracted from formalin-fixed, paraffin-embedded (FFPE) tumor samples
- Employs single DNA extraction method from FFPE specimens, 30-200 ng of which undergoes multiple steps including fragmentation, end repair and adenylation, adapter ligation, library construction/amplification, hybridization and capture, sequencing and data analysis
EFFECTIVENESS & SAFETY:
- Clinical test as an aid to clinicians in identifying ovarian cancer patients who may be eligible for treatment with Zejula (niraparib)
- Primary endpoint: Overall Response Rate (ORR), key secondary endpoints were ORR in all patients and Median DOR (duration of response) in all patients
- In patients with tBRCAm tumors (n=63), ORR was 28.6% and median DOR 9.2 mo.
- Statistically significant overall response rate with clinically meaningful DOR seen in patients with deleterious or suspected deleterious BRCA mutation, positive GIS status, patients who had received prior chemotherapy
- Overall, the response rate is better than what would be expected of available therapy and represents an improvement in a surrogate endpoint that is reasonably likely to predict clinical benefit
- Risks are associated with potential mismanagement of patients resulting from false results of the test or a failure to receive results
REGULATORY PATHWAY: PMA
- Device Generic Name: Next Gen Sequencing oncology panel, somatic or germline variant detection system
- Device Procode: PQP
Patient Labeling: Test Request Form
 PENTAX Medical Video Duodenoscope ED34-i10T2
PENTAX Medical Video Duodenoscope ED34-i10T2
Pentax
INDICATION FOR USE: To provide visualization and access to the upper gastrointestinal (GI) tract to treat bile duct disorders and other upper GI problems
ADDRESSING UNMET NEED: First duodenoscope with disposable elevator piece, reducing the number of parts needing disinfection. Represents a major step toward lowering the risk of infection among patients who undergo procedures with these devices
DEVICE DESCRIPTION:
- Intended to be used with endoscopic devices, introduced in patient’s mouth
- Provides visualization via a video monitor of and therapeutic access to the biliary tract (liver, gall bladder and bile ducts) through the upper gastrointestinal tract
- Risks: Ootential for injuries, including, but not limited to, burns, electric shock, perforation, infection and bleeding
REGULATORY PATHWAY: 510(k)
 MISIGHT (omafilcon A) contact lens
MISIGHT (omafilcon A) contact lens
Cooper Vision
INDICATION FOR USE: Single use Soft Contact Lenses for the correction of myopic ametropia and for slowing the progression of myopia in children with non-diseased eyes, who at the initiation of treatment are 8-12 years of age and have a refraction of -0.75 to -4.00 diopters (spherical equivalent) with ≤ 0.75 diopters of astigmatism.
The lens is to be discarded after each removal
ADDRESSING UNMET NEED: First contact lens indicated to slow the progression of myopia (nearsightedness) in children, which ultimately could mean a reduced risk of developing other eye problems
EFFECTIVENESS & SAFETY:
- Three-year randomized, controlled clinical trial , n=135 children ages 8 to 12, MiSight vs. conventional soft contact lens
- Progression in myopia with MiSight lenses less than conventional lenses, less change in the axial length of eyeball at each annual checkup
- No serious ocular adverse events in either arm of the study
- Additionally, real world data from a retrospective analysis of n-782 medical records from seven community clinics. Ulcer rate comparable to adults who wear contact lenses daily
- Required postmarket study to further evaluate the safety and effectiveness
REGULATORY PATHWAY: PMA
- Classification name: Daily Wear Soft Contact Lens To Reduce The Progression Of Myopia
- Product Code: QIT
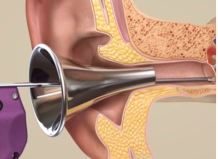 TULA System
TULA System
Tusker Medical
INDICATION FOR USE: Tubes Under Local Anesthesia (TULA) System for delivery of tympanostomy tubes, that can be inserted into the eardrum to treat recurrent otitis media.
ADDRESSING UNMET NEED: An option for the treatment of recurrent ear infections that does not require general anesthesia. Has the potential to expand patient access to treatment that can be administered in physician’s office with local anesthesia and minimal discomfort
DEVICE DESCRIPTION:
- Consists of an Iontophoresis System, a local anesthetic, and a Tube Delivery System
- Iontophoresis System is designed to provide child-friendly bilateral local anesthesia of the tympanic membrane, enabling a viable alternative to general anesthesia
- Once the tympanic membrane is numb, the Tube Delivery System is used to insert the tympanostomy tube
- With a single button push, the Tube Delivery System automatically creates the myringotomy and inserts the tube in less than 500 milliseconds
- Parents are present during the TULA office-based procedure, and younger children may sit on their parent’s lap, if desired.
EFFECTIVENESS $ SAFETY:
- Study with 222 pediatric patients to assess the effectiveness of the Tula System for the delivery of ear tubes
- Procedural success rate was 86% and 89% in children younger than age 5 and between ages 5-12 years old, respectively
- Most common adverse event: Inadequate anesthesia during the procedure.
REGULATORY PATHWAY: PMA
- Breakthrough Device designation
Image Credit:Myriad Genetic Laboratories, Pentax, Cooper Vision, Tusker Medical