COVID-19 News: Janssen vaccine pause Q/A, Vaccine quality, #VaccineReady campaign, 2020 CDRH innovation, Bamlanivimab EUA revoked, Pooling and serial testing of diagnostics, Remote interactive inspections
J&J Vaccine Pause Q&A
The FDA and CDC are reviewing data involving cases of a low level of platelets in combination with a rare and severe type of blood clot – cerebral venous sinus thrombosis (CVST)
- Out of an abundance of caution, the FDA and CDC are recommending a pause in J&J vaccines
- The agencies are investigating all reports to determine a causal relationship
- The agencies are ensuring health care providers for proper symptom recognition and management due to the unique treatment required for this condition
- For more information, visit this CDC page
Important Steps to Ensure Quality, Safety and Effectiveness of Authorized COVID-19 Vaccines
FDA’s inspections review the quality of manufacturing procedures, including records, staff training, facility operations, drug production and testing and the systems in place to ensure product quality
- During an inspection of Emergent BioSolutions, production site for J&J vaccine, a number of concerning observations (Form 483)
- Pause new vaccine production while issues are resolved
- For vaccines already manufactured, additional testing before distribution
#VaccineReady campaign
FDA’s Office of Minority Health and Health Equity has joined the U.S. DHHS #VaccineReady campaign to address vaccine disparities
- Developed social medial messages to encourage vaccination of diverse communities
- Twitter and Facebook messages with a variety of graphics and videos on topics such as vaccine development, vaccine authorization, and the benefits of getting vaccinated against COVID-19
Twitter Graphics: Download and Share
Facebook Graphics: Download and Share
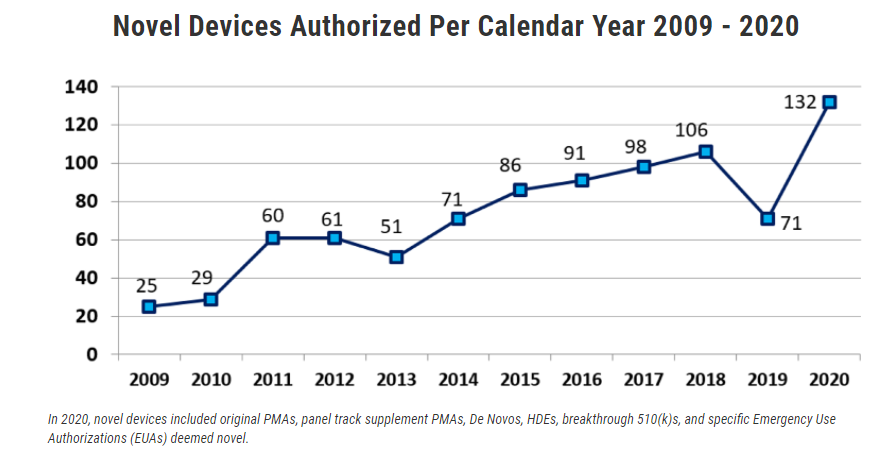
2020 CDRH innovation to combat pandemic
High volume of EUAs including those for novel devices
Remarkable ability of the device ecosystem and the FDA to adapt and respond to emerging public health needs
- First sample pooling in diagnostic testing for COVID-19
- First Test for Screening of People Without Known or Suspected COVID-19 Infection
- First Combination Diagnostic Test for detection and differentiation of the viruses that cause flu and COVID-19
- First diagnostic test where results can be read directly from testing card
- First point-of-care (POC) tests:
- Home use tests:
Bamlanivimab EUA revoked
Revoked EUA for bamlanivimab, when administered alone, for treatment of mild-to-moderate COVID-19 in adults and certain pediatric patients
- Ongoing analysis of emerging scientific data, specifically the sustained increase of variants resistant to bamlanivimab alone
- Other monoclonal antibody therapies authorized for EUA remain appropriate treatment choices
Streamlining Pooling and Serial Testing for Certain Molecular Diagnostic Tests
Amendment to allow certain authorized molecular diagnostic tests to be distributed and used to pool anterior nasal respiratory specimens from asymptomatic individuals
- Prior to a test being distributed or used for any new indication, the developer must submit a notification to the FDA with the information required by the amendment
Remote Interactive Inspections of Drug Manufacturing and Bioresearch Monitoring Facilities
Use of various interactive and virtual tools for remote interactive evaluation of manufacturing facilities
- Will use existing risk management methods and related tools to determine remote interactive evaluation
- For pre-approval and pre-license, post-approval, surveillance, for-cause and bioresearch monitoring programs
Image credit: FDA. CDC. Emergent, Lilly