Marketing Authorizations: ABECMA for multiple myeloma, PoNS for gait in multiple sclerosis, HARMONY for pulmonary valve regurgitation, Q-COLLAR for brain injury protection, ClearUP for sinus pain

ABECMA (idecabtagene vicleucel) injection
Bristol-Myers Squibb
INDICATION: B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an antiCD38 monoclonal antibody
USE: Each dose of Abecma is a customized treatment created by using a patient’s own T-cells which are collected and genetically modified to include a new gene that facilitates targeting and killing myeloma cells. Once the cells are modified, they are infused back into the patient.
ADDRESSING UNMET NEED: First cell-based gene therapy for adult patients with multiple myeloma
MECHANISM OF ACTION: Chimeric antigen receptor (CAR)-positive T cell therapy targeting B-cell maturation antigen (BCMA), expressed on normal and malignant plasma cell surfaces. Antigen-specific activation of ABECMA results in CAR-positive T cell proliferation, cytokine secretion, and subsequent cytolytic killing of BCMA-expressing cells
EFFICACY:
- Open-label, single-arm, multicenter study in adults (n=127) with relapsed and refractory multiple myeloma who had received at least 3 prior lines of antimyeloma therapy
- Endpoints: Overall response rate (ORR), stringent complete response (sCR) rate, Very good partial response (VGPR), duration of response (DOR) based on the International Myeloma Working Group (IMWG) Uniform Response Criteria for Multiple Myeloma
- ORR=72%, sCR=28%, VGPR=25%, PR=19%, median DOR (PR or better)= 11 mo.
SAFETY:
- Boxed warning: Cytokine release syndrome (CRS), hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS), neurologic toxicity, and prolonged cytopenia, all of which can be fatal or life-threatening.
- Most common side effects: CRS, infections, fatigue, musculoskeletal pain, and a weakened immune system
- REMS: Certified staff involved in the prescribing, dispensing or administering of Abecma trained to recognize and manage CRS and nervous system toxicities and other side effects
REGULATORY PATHWAY: BLA
- Orphan Drug and Breakthrough Therapy designations
- Post-marketing observational safety study involving patients treated with Abecma
Portable Neuromodulation Stimulator (PoNS)
Helius Medical
INDICATION FOR USE: Short-term treatment of gait deficit due to mild to moderate symptoms from multiple sclerosis
ADDRESSING UNMET NEED: New aid in physical therapy for those who live with MS on a daily basis
DESCRIPTION: Portable, non-implantable delivery of mild neuromuscular electrical stimulation to dorsal surface of tongue
- Consists of controller and mouthpiece connected to each other by a cord
- Mouthpiece is held lightly in place by the lips and teeth and control unit is worn around the neck
- Controller sends signals to mouthpiece; receptors on tongue send millions of neural impulses to the brain
- Additionally, therapist can connect the control unit to a computer and view usage data to improve a patient’s therapy by identifying potential areas of missed or shortened sessions
EFFECTIVENESS & SAFETY:
- Two clinical studies and a retrospective analysis of real-world data (RWD)
- First Study: Randomized, double blind controlled trial (n=20 MS patients), PoNS vs sham control : Dynamic Gait Index (DGI) at 2, 6, 10, 14 weeks showed statistically and clinically significant imporvement with PoNS
- Second Study: Randomized controlled trial (n=14 MS patients). PoNS vs sham control : Effect on cognitive and physical rehab. Sensory Organization Tasks (SOT) and DGI scores at 14 weeks showed significant improvement
- RWD study: Retrospective analysis of patient feedback Functional Gait Assessment in clinical rehabilitation settings
- Safety: Prescription use only in accordance with warnings and precautions (inducing electrical safety) in labeling
REGULATORY PATHWAY: De Novo, Breakthrough designation
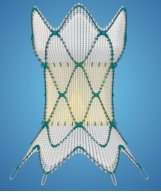
HARMONY Transcatheter Pulmonary Valve (TPV) System
Medtronic
INDICATION FOR USE: To improve blood flow to the lungs in patients with severe pulmonary valve regurgitation without open-heart surgery, which is the current standard of care. Patients have a native or surgically-repaired right ventricular outflow tract (RVOT). The use of the Harmony valve may delay the time before a patient needs additional open-heart surgery. It can also potentially reduce the total number of open-heart surgeries required over an individual’s lifetime.
ADDRESSING UNMET NEED: New less-invasive treatment alternative to open-heart surgery for adult and pediatric patients with certain types of congenital heart disease. May help patients improve their quality of life and return to their normal activities more quickly.
DESCRIPTION: Pulmonary Valve Prosthesis percutaneously delivered
- Intended to replace patient’s pulmonary heart valve; different from heart valves in that they are placed percutaneously and do not require open chest surgery or a cardiotomy for placement
- During the implantation procedure, a thin, hollow tube (catheter) with a collapsed Harmony valve on the end is inserted through a vein in the groin or in the neck and into the right side of the heart, and then into the RVOT where it is placed into position
- Valve is then released from the catheter; it expands on its own, and anchors to the RVOT
- Once new valve is in place, it opens and closes like a door to force the blood to flow in the correct direction
EFFECTIVENESS & SAFETY:
- Prospective, non-randomized, multi-center clinical study, n=70 patients
- Follow-up examinations at study start, at implant procedure, discharge, and post implant at one month, six months, and annually through five years
- Primary effectiveness endpoint: 89.2% patients had no additional surgical or interventional procedures related to the device and acceptable heart blood flow function at six month
- Primary safety endpoint: No procedure- or device-related death within 30 days following implant
- Adverse events: Irregular or abnormal heart rhythm, ventricular tachycardia), leakage around the valve, minor bleeding, narrowing of the pulmonary valve, movement of implant
REGULATORY PATHWAY: PMA
- Breakthrough Device Designation
- Part of the U.S.-Japan Medical Device Harmonization by Doing Collaboration, to promote timely access to innovative devices in both the U.S. and Japan
- Post-approval study with 10 years followup
Q30 Sports Science
INDICATION FOR USE: Non-invasive device intended to be worn around the neck of athletes aged 13 years and older during sports activities to aid in the protection of the brain from effects associated with repetitive sub-concussive head impacts. For OTC use.
ADDRESSING UNMET NEED: Protective equipment for athletes when playing sports to help protect their brains from the effects of repetitive head impacts
DESCRIPTION:
- C-shaped collar that provides compressive force to the internal jugular veins, which in turn increases the blood volume in the skull’s blood vessels
- Blunt trauma accidents cause brain moves unrestrained in the skull – “slosh”
- Q-Collar’s increase in blood volume in those blood vessels creates a tighter fit of the brain inside the skull and reduces the “slosh” movement
- By reducing brain movement within cranial space, Q-Collar may aid in protection from effects of head impacts
EFFECTIVENESS & SAFETY:
- Prospective, longitudinal study, n=284 subjects 13 years or older from a high school football team, Q-collar vs no collar; all wore accelerometer device that measured every head impact during play
- Diffusion Tensor Imaging (MRI type) of brain to compare structural changes pre-season and post-season
- Significant deep tissues changes (involved in transmission of electrical nerve signals) in 73% no-Collar group vs. 13% in Q-collar group
- No significant adverse events with device use
REGULATORY PATHWAY: De Novo classification
ClearUP Sinus Relief
Tivic Health Systems
INDICATION FOR USE: Transcutaneous electrical nerve stimulator that electrically stimulates the skin overlying the paranasal sinuses and is intended to be used for the temporary relief of moderate to severe congestion. ClearUP Sinus Relief is a treatment to be used at home by individuals 18 and older
ADDRESSING UNMET NEED: OTC availability of device for sinus pain treatment
DESCRIPTION: Transcutaneous electrical nerve stimulator for the relief of congestion.
- Electrically stimulates the skin overlying the paranasal sinuses to relieve congestion
REGULATORY PATHWAY: De Novo reclassification
Image credit: Bristol-Myers Squibb, Helius Medical, Medtronic, Q30 Sports, Tivic Health