Expediting Medical Device Regulatory Review and Reimbursement
FDA Brief: Week of Feb 22, 2016
Proposal for Joint FDA-Coverage Organization Meeting to discuss Evidentiary Standards for Regulatory Review and Reimbursement
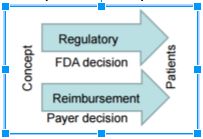

- 2011-2015: FDA-CMS Pilot Initiative for parallel discussion and review of device premarket clearance (by FDA) and National Coverage Determination (by CMS)
- 2016: FDA intends to provide a mechanism to get early payer input on evidentiary needs to streamline process from FDA Approval to Payer Coverage
- Use CDRH’s Pre-Submission program as a forum for joint discussion between Sponsor, FDA and Coverage organization